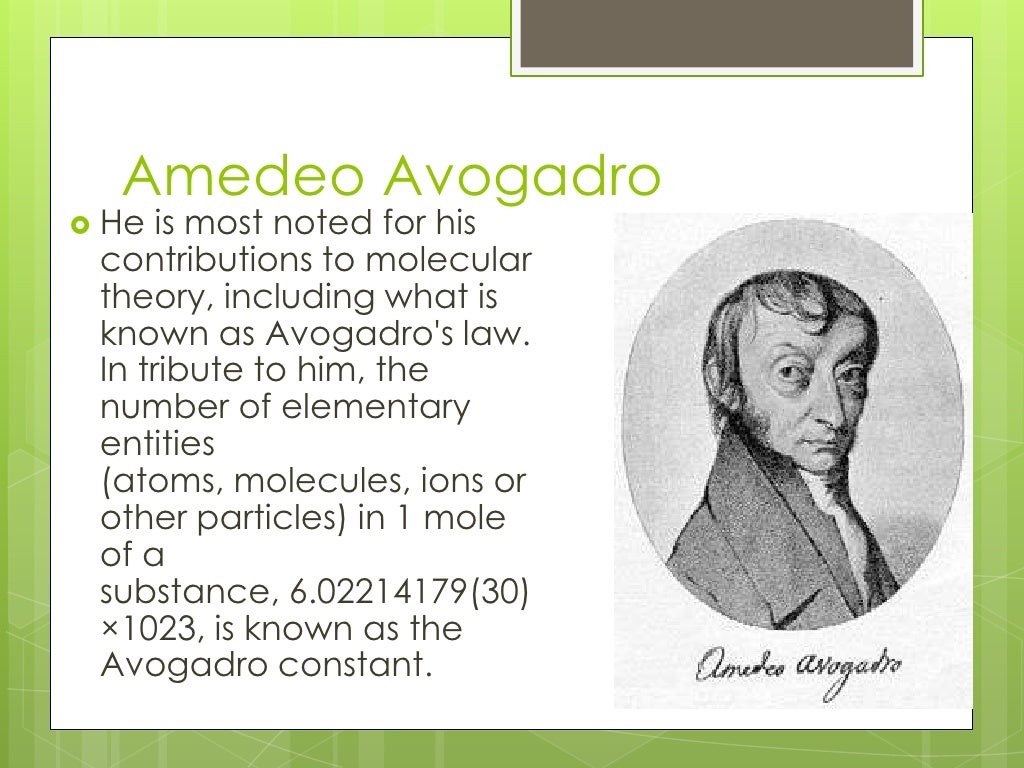
Lornezo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto was a famous creation scientist best known for his contributions to chemistry. He was born in Turin, and his family was also well-known as a lawyer in Italy. He fame is derived from his contribution about molecular law, known as Avogadro's law and Avogadro's number. Also, he has taught many scientific theories to his students. [1]
Both Josef Loschmidt and Amedeo Avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. Neither scientist discovered “Avogadro’s number” in the form we use it today (6.02 x 10 23). Still, there’s a controversy over the name. Research the contributions from these two scientists and read about how Avogadro’s number got its name. July 9, 1856) - better known as Amedeo Avogadro -was an Italian scientist born in the Kingdom of Sardinia ad Piedmont, most noted for his contributions to the theory of molarity and molecular weight. The number of molecules in one mole is called Avogadro's number is honor of him, as is Avogadro's law. The contributions of the Italian chemist Amedeo Avogadro (1776–1856) relate to the work of two of his contemporaries, Joseph Louis Gay-Lussac and John Dalton. Gay-Lussac’s law of combining volumes (1808) stated that when two gases react, the volumes of the reactants and products—if gases—are in whole number ratios.
Biography

He was born August 9, 1779 in Turin, Italy. He was a very famous Italian scientist. He wanted to follow his family's steps and then so he decided to enter into the study of ecclesiastical law. [2]. He had achieved success as an ecclesiastical lawyer; he had a interest in a variety of sciences like natural philosophy, mathematics, and physics at that time. Therefore, he was selected as the demonstrator in Turin and started to teach his students in college. [3]. He gained many achievements as a chemist and then retired in 1850. His working must be contributed increasing of scientific development today. He wanted to follow his father's step and then he could be a famous chemist in science.
LawyerAvogadro was born by Count Filippo Avogadro and Anna Maria Verellone. He close affection with his family led him into a legal career. His father was famous lawyer and he also became a lawyer in Turin. As a lawyer, he used to teach his students and he struggled to increase education at that time. In spite of his lawyer training, he had lots of interests about science and then he decided to became a chemist. He contributed to develop increasing of scientific innovation[4].
Avogadro's law
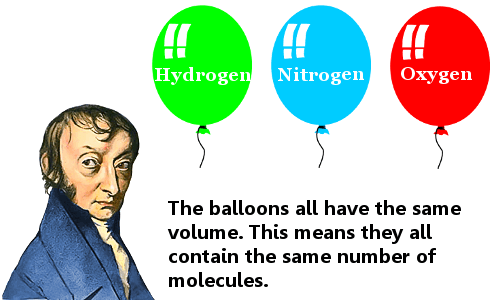
Avogadro's law isscientific law regarding the nature of chemical changes. The law states that for volumes of gases with equal temperatures and pressures, they have the same number of molecules.[5]. Although his laws were not considered important until 1858, he claimed that particles could be combined of molecules throughout his hypothesis at that time. Particles were formed by units and atoms. It was declared to explain gas reactions by Avogadro. It can be shown by V/N=K. The important thing was that the gas had equal value gases. Furthermore, a mole is a unit which is the amount of substance contained in 6.022x1023. Therefore, Avogadro's number is known as 6.022x1023 molecules/gram. The size of number is hard to understand and it is a huge number. It was determined by the mole.[6]. It brought many affected results such as Boyle's law, Charles's law, and Gay-Lussac's law. It also contributed to cause scientific development. [7] Sonic r online, free no download.
ContributionsAmedeo Avogadro had remained many contributions as a chemist in the world. He had been experienced pains, obstacles, and adversity during his life. Especially, he helped to claim a variety of laws to help developing of science at that time.[8]. For example, Avogadro’s law is one of the important hypothesises when he declared at that time. It is the relationship whit volume of gases, same temperature and pressure, and molecular weight. Its used to be neglected until it was portrayed by Stanislao Cannizarro. It was shown the book in the world.[9]. Also, he declared an article about Journal de physique in 1811. It represents differences of the molecules and atom throughout his experiments. He defined that the word “mole” to mean the smallest compounds. It was the motivation to know how he was great chemist at that time.

Amedeo Avogadro Law

Nineteenth-CenturyThere were a lot of scientists in Nineteenth-century; they were John Dalton,Copernicus, Kepler, Galileo, Newton, and Amedeo Avogadro. They had brought many laws to increase scientific development at that time. They caused many changes to make development at that time and then they accomplished growth rapidly.[10] Avogadro was one of the people who contributed to help growth of science. Office 2016 for mac system requirements. Avogadro's molecular theory was one of the representative laws. [11]
Video
Amedeo Avogadro Law Equation
Amedeo Avogadro (Avogadro's law) Epson xp 342 driver for mac.
References
- Amedeo Avogadro Copyright ©2010 Soylent Communications,NNDB
| ||||||||||||
Amedeo Avogadro Invention
